GxP Technologies
Digital Cloud & AI System Implementation

USDM Life Sciences has built its brand on core qualifications and validation services for pharma, biotech, and medical device companies. But we don't stop there.
Your trusted partner for Digital Cloud & AI system implementation, we provide end-to-end services so that GxP and non-GxP customers build and implement the compliant systems they need to successfully compete in the industry.
There are three categories of services under the umbrella of USDM System Implementation services: 1) cloud platform services, 2) cloud data and AI services, and 3) cloud cybersecurity, risk management, and compliance. Each includes support and maintenance activities performed by our technology experts.
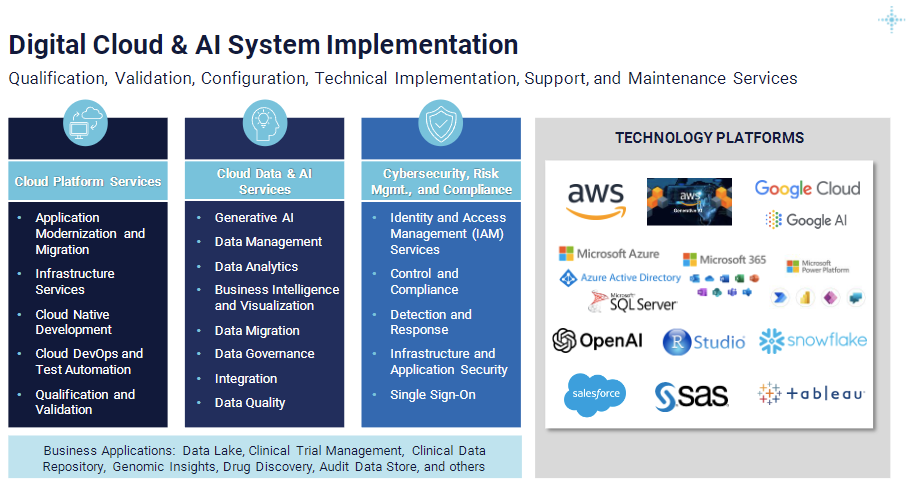
Cloud Platform Services
- Application Modernization and Migration. Boost scalability, performance, and reliability with USDM’s state-of-the-art solutions for cloud environments. We guarantee cost-effectiveness through contemporary and innovative methodologies.
- Infrastructure Services. Experience the future of infrastructure management. USDM provisions virtualized computing resources—like servers, storage, and networking—across cloud environments to help you manage your infrastructure with unparalleled flexibility and cost efficiency.
- Cloud Native Development. Stay ahead in the digital era with USDM’s expertise in cloud native development. We craft applications for dynamic and distributed cloud computing platforms. Our approach leverages modern technologies (i.e., containers, microservices, and serverless functions) to ensure that your applications thrive in elastic environments.
- Cloud DevOps and Test Automation. Improve operational efficiency with USDM’s forward-thinking cloud DevOps and test automation solutions. Our approach integrates development and operations teams to bring innovation to the forefront and enhance collaboration and productivity. It also automates the software delivery process using robust features like continuous integration and continuous delivery (CI/CD) pipelines, infrastructure as code (IaC), and advanced automated testing.
- Qualification and Validation. Choose USDM as your trusted partner for cloud solutions that exceed industry expectations and regulatory requirements. In sectors as critical as healthcare and finance, our qualification and validation processes provide a robust framework that assures customers of its reliability, security, and compliance.
Cloud Data and AI Services
- Generative AI. Explore unparalleled possibilities with USDM Cloud Data and AI services, where creativity meets artificial intelligence for transformative outcomes. USDM is pioneering the integration of advanced AI models in life sciences organizations to help them generate dynamic content with text, images, and more. Our generative AI services are finding applications in content creation, design enhancements, and decision support systems.
- Data Management. Elevate your data experiences with technology and unparalleled reliability in the cloud. USDM is striving to revolutionize data handling, storage, and retrieval in cloud environments to guarantee seamless accessibility and unwavering data integrity. Our advanced data management solutions redefine efficiency to ensure that your business stays at the forefront of data-driven innovation.
- Data Analytics. Make data-driven decisions and pave the way for your future success. We are leading the way in capturing, managing, and analyzing data to propel your business strategy and performance to new heights. Our data analytics solutions offer an analysis of past events and a forward-looking scenario for planning and predictive modeling.
- Business Intelligence and Visualization. Be at the forefront of innovation by translating insights into actionable strategies for unprecedented business growth. Explore our groundbreaking BI solutions, empower your entire organization with self-serve data capabilities, and avoid the chaos of unstructured information.
- Data Migration. Optimize your digital transformation journey by redefining efficiency, reliability, and business continuity. USDM accelerates data migration by seamlessly transferring data from various sources to your cloud environment. Our approach involves meticulous planning to guarantee data integrity and minimize downtime.
- Data Governance. Elevate your data strategy with security, compliance, and business resilience. In an era of voluminous data, companies are eager to make use of it. But lax governance, data breaches, ransomware, and unforeseen data loss introduce the potential for reputational risks. USDM developed a transformative approach that emphasizes the importance of reliable data underpinned by robust governance.
- Integration. Redefine efficiency and agility in your business operations. USDM is perfecting the art of integration by seamlessly connecting legacy systems with modern cloud services. Our approach ensures a harmonious data flow and optimal functionality across your organization’s entire IT landscape.
- Data Quality. Explore the transformative impact of superior data quality. When precision, potential, and every data point contribute to a strategic advantage, USDM ensures data quality for accurate analysis, process automation and efficiency, and growth opportunities. Similarly, we ensure that poor-quality data does not put your business at risk.
Cloud Cybersecurity, Risk Management, and Compliance
- Identity and Access Management Services. Experience a transformative blend of robust security measures and user-friendly access management. Our tools and protocols efficiently manage user identities and access rights across diverse cloud services, elevate security standards, and enhance user convenience.
- Control and Compliance. Improve your security posture with USDM’s forward-thinking solutions, where proactive measures and transparency converge for unparalleled cybersecurity resilience. We lead the charge in securing cloud environments and guarantee adherence to stringent regulatory requirements using a comprehensive framework. It includes regular audits, rigorous policy enforcement, and robust reporting mechanisms.
- Detection and Response. Embrace a future where security is not just a measure, but a continuous proactive strategy. We help you to meticulously monitor your cloud environments and swiftly identify security threats and anomalies. Our advanced capabilities enable prompt response and mitigation of detected incidents to effectively safeguard your sensitive information.
- Infrastructure and Application Security. Elevate your defense strategy with USDM to ensure that your digital assets are protected against external and internal cybersecurity threats. Our comprehensive security measures include cutting-edge encryption, robust firewalls, and proactive vulnerability assessments.
- Single Sign-On. Redefine the user access paradigm with USDM, where technology meets a heightened sense of security and enhances the user journey. USDM offers a revolutionary access management component in our solution for secure user access to protected applications. We enable frictionless and risk-based authentication, a least privilege policy, and the seamless experience of Single Sign-On (SSO).
Case Studies
Improved Transparency and Compliance for Clinical Data Lake
Learn how USDM helped data scientists perform unstructured data analysis using the AWS S3 and AWS Lake Formation services.
Unique Architecture Blueprint Supports High Performance Computing
Learn how the USDM team helped to build and validate the architecture that enabled AWS based infrastructure-as-code, AWS DataSync, and the Amazon Elastic MapReduce (EMR) tool for big data processing and analysis.
USDM Designs AWS Data Lake to Standardize GxP Data Management Processes
Learn how USDM helped a global biotechnology company reduce maintenance, security, and compliance costs by implementing a single centralized data platform that is GxP and GDPR-compliant.
Schedule a consultation to learn more about our system implementation services!
Related Services
USDM Cloud Assurance
USDM Cloud Assurance is a managed service that offloads your SaaS vendor management and maintenance of ongoing system updates, patches, and changes.
ProcessX
ProcessX is an intelligent, fully validated, GxP process automation platform built on ServiceNow for all those processes that still have you pushing paper.
Cloud Managed Services
USDM’s managed services for life sciences are bundled into subscription models or offered at fixed fees to control your IT spending while maintaining compliance.
Resources that might interest you

- 0
- Various Authors
- -

- Stepheni Norton
- -

- 0
- Various Authors
- -

- 0
- Kim Hutchings
- -

- 0
- Vishal Sharma
- -

- 0
- Stepheni Norton
- -
USDM Thought Leaders




